Theme: Healthcare Policy-Makers And Payers: Key Concerns In Paradigm Shift Of Pharma Industry Trends
Pharma Congress 2017
ConferenceSeries Ltd invites all the participants across the globe to attend 12th World Pharma Congress during October 16-18, 2017 Budapest, Hungary. Which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Pharma Congress 2017 is a specially designed cluster Pharma conference. The main theme of this Pharma conferences is “Current Technologies & Advancements In Pharmaceutical Field’’. The Impact of developing and Marketing Drugs, its Registration & Approval” which covers a wide range of critically important sessions.
Why to Attend???
Meet Your Target Market with members from around the world focused on learning about Pharma, this is your single best opportunity to reach the largest assemblage of participants from the Pharma community. Conduct demonstrations, distribute information, meet with current and potential Scientists, make a splash with a new research, and receive name recognition at this 3-day Pharmaceutical Event.World-renowned speakers, the most recent techniques, tactics, and the newest updates in Pharmaceutical Sciences fields are hallmarks of this conference.
A Unique Opportunity for Advertisers and Sponsors at this International event:
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneur
With the evolving European pharmaceutical Market Pharma Congress 2017 aspires to provide a significant platform for Pharmacy and Pharmaceutical sciences interdisciplinary subjects to explore the theme of “Healthcare Policy Makers and Payers Key Concerns in Paradigm Shift of Pharma Industry Trends” will emphasize the diversified perspective of pharma R&D investment, formulation, regulatory approvals and marketing.
Track 1: Pharmaceutical Sciences
Disease is the enemy of mankind, which affects part or all of organism. Efforts to cure or improve the health condition against disease lead a remarkable improvement in the field of pharma. Pharmaceutical science is a wide range of combinations of scientific disciplines that contribute to the discovery and development of new drugs and pharmacotherapies. Advancements in the branches of pharmaceutical sciences enhance the development of new reliable pharma entities. Every aspect of the pharmaceutical science deals with the design, action, delivery, and disposition of drugs. The Economist Intelligence Unit (EIU) reports that health care spending in the 60 countries that it covers rose by 2.6 percent in nominal U.S. dollar terms in 2014. Global health care spending is expected to increase by an average of 4.3 percent in 2015-2019. And the Size of the pharma market in EU set to grow by 27% between 2015- & 2022.
9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 5th International Pharmacy Conference, September 01-02, 2017 Las Vegas, USA5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 6th FIP Pharmaceutical Sciences World Congress 2017, Stockholm, May 2017, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
International Biopharmaceutical Association, European Biopharmaceutical Enterprises, Pharmaceutical Society of Australia, Canadian Pharmacists Association, Pharmaceutical Group of the European Union (PGEU), AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 2: Pharmaceutical Analysis
Producing safe and effective pharmaceutical formulation is the main goal for Pharmaceutical industry. Hence pharmaceutical industry has great concern towards quality and quantity to develop pure and safe pharma products. The trends in pharmaceutical analysis focuses on the modern methods, technology and tools for control of related impurities, and faster analysis time, better separation time and faster method development.
Related Pharmaceutical Events| Pharmaceutical Conferences
International Conference on Pharmaceutical and Biomedical Engineering, June 12-13, 2017 Taipei, Taiwan, International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, 10th Asia-Pacific Pharma Congress May 08-10, 2017 Singapore, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE,
Related Societies
Applied Pharmaceutical Analysis, Joint Pharmaceutical Analysis Group, Association of Clinical Research Professionals - ACRP, Association of Clinical Research Organizations, European Pharmaceutical Union, International Pharmaceutical Federation (FIP) AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 3: Drug Discovery and Research
Continuous innovative development is one of the eminent characters of pharmaceutical industry. Pharmaceutical innovation is an organized, conceptual process. comprehensive research is resulting in an enhanced method of drug discovery and development. The invention of new medicines and the improvement of existing drugs constitute the development firms in pharma industry. The periodic achievement of developing novel therapy in an area with no prior treatments counts among the pharmaceutical industry's most defining characteristic. For instance, in the USA, the total number of new drugs approved between 2000 and 2010 was only 333, which seems surprisingly low. The Centre for Medicine Research International (CMR) reported in its Pharmaceutical R&D Fact book 2014 an average success rate of 4.9 % from first toxicity dose to market approval with between phase success rates of 66, 44, 26, 72 and 91 % from first toxicity dose to first human dose.
Related Pharmaceutical Events| Pharmaceutical Conferences
International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 5th International Conference on Clinical Pharmacy, October 23-24, 2017 Orlando, Florida, USA, 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
National Pharmacy Association, Drug Discovery and Development Interface (DDDI), Royal Pharmaceutical Society (RPS), International Pharmaceutical Federation (FIP), European Association of Employed Community Pharmacists in Europe (EPhEU), AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 4: Drug Therapy
Pharmacotherapy deals in administering pharmaceutical drugs distinguishing from therapy using surgery, radiation, movement or other methods. Drug therapy complies patient care with various types of pharmaceutical drugs ensuring the safe, appropriate and economical use. North America is expected to account for more than half the therapeutic drug monitoring market share in 2015. It is the prime market for therapeutic drug monitoring. The market in Europe on the other hand is growing at a slower rate. Therapeutic Drug Monitoring Market’s worth is expected 2.55 Billion USD by 2020 globally.
Related Pharmaceutical Events| Pharmaceutical Conferences
10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK, International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
Therapeutic Goods Administration (TGA), American Tinnitus Association, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Pharmaceutical Group of the European Union (PGEU), European Association of Employed Community Pharmacists in Europe (EPhEU),
Track 5: Drug Delivery Systems
To obtain a required therapeutic effect, type of formulation, technology and system for transporting drug are the important variables. Drug delivery technologies alter the drug release profiles, absorption, distribution, and elimination of product for the safety efficacy. Developing new avenues for drug delivery vehicles reported promising results. The practice of drug delivery has changed dramatically in the past few decades and even greater changes are anticipated in the near future. Thus in 2012 BCC Research estimates the overall market for drug delivery systems including both large and small molecule drugs was $147 billion and expects it will reach $175.1 billion by 2018
Related Pharmaceutical Events| Pharmaceutical Conferences
10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK, 3rd International Conference and Expo on Drug Discovery & Designing, September 25-27, 2017, Vienna, Austria, International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE
Related Societies
International Pharmaceutical Federation (FIP), European Association of Employed Community Pharmacists in Europe (EPhEU), European Pharmaceutical Union, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 6: Advances In Clinical Trials
Clinical trials are the clinical investigation of a drug that administered or dispensed to, or used involving one or more human subjects. Clinical trials are the major parameter for the approval of drug, as they mainly deals with the effectiveness and safety. Clinical trials play a vital role in the development of new drug entity as it subjected to various phases for approval. Advancements in clinical trials enhance the drug development process by means of reducing time duration, sometimes the cost. The success rate of Phase II trials that proceed to Phase III, as of 2010, is 18%. The amount of money spent on Phase II/III trials in 2015 by Large Sponsors (R&D $500M+) was $465,725,000 on average, while Non-Large Sponsors (R&D <$500M) spent $13,352,000 on average. The entire process of a drug from lab to this point may take approximately 12 to 18 years (but not always), often costing over $1 billion.
Related Pharmaceutical Events| Pharmaceutical Conferences
5th International Conference on Clinical Pharmacy, October 23-24, 2017 Orlando, Florida, USA, 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 Chicago, Illinois, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, European Biopharma Congress, November 16-17, 2017 Vienna, Austria, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA
Related Societies
Association of Clinical Research Professionals - ACRP, International Biopharmaceutical Association, European Biopharmaceutical Enterprises, Association of Clinical Research Organizations, European Pharmaceutical Union, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association
Track 7: Pharmaceutical Formulations
Pharmaceutical formulations is extensive procedure involving characterization of a drug's physical, chemical, and mechanical properties in order to choose what other ingredients (excipients) should be used in the preparation and resulting a safe and efficacy product. Modern pharmaceutical formulation is expanding its sector with technology and innovation to formulate eminent result oriented formulations. The global pharmaceutical excipients market is projected to reach USD 8.1 Billion in 2021 at a CAGR of 6.1% in the forecast period 2016 to 2021. The rising demand for new drug delivery systems, greater understanding of the functional benefits of excipients, growing pharmaceutical industry, and patent expiries of several blockbuster drugs are positively impacting the overall growth of the market.
Related Pharmaceutical Events| Pharmaceutical Conferences
International Conference on Pharmaceutical and Biomedical Engineering, June 12-13, 2017 Taipei, Taiwan, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore
Related Societies
European Association of Employed Community Pharmacists in Europe (EPhEU), European Pharmaceutical Union, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), International Pharmaceutical Federation (FIP), Drug Discovery and Development Interface (DDDI)
Track 8: Innovations In Drug Development
Drug development involves enormous methods and development procedure to attain therapeutic effective level. Emerging new Formulation is a persistent process with utilisation of abundant knowledge of pharmaceutics. Considering all characteristics of substances used, and their behaviour towards formulations need more innovative approach of drug development. Approximately 1,200 biopharma companies in the United States, more than 90 percent of these enterprises do not earn a profit and focus on innovative R&D for future products.
Related Pharmaceutical Events| Pharmaceutical Conferences
9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom,10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore, 7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE
Related Societies
International Pharmaceutical Federation (FIP), European Association of Employed Community Pharmacists in Europe (EPhEU), European Pharmaceutical Union, Pharmaceutical Society of Australia, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Regulatory Affairs Professionals Society (RAPS), Association of Clinical Research Organizations
Track 9: Regulatory Affairs & Intellectual Property Rights
Regulatory Affairs has a very specific meaning within the healthcare industries. Its regulations are for systematic manufacturing and marketing of safe, efficacious and qualitative drugs. Improving new regulations and adapting to the technology regulatory affairs set new goals and challenges to the pharmaceutical formulation. The global regulatory affairs outsourcing market had a valuation of US$1.9 bn in 2014. The market is expected to expand at a substantial 11.5% CAGR from 2015 to 2023 and rise to a valuation of US$5.7 billion by 2023.
Related Pharmaceutical Events| Pharmaceutical Conferences
Industrial Pharmacy Conference April 11-12, 2016 Dubai, UAE, 12th Annual Pharma Middle East Congress, Sep 25-27, 2017 Dubai, UAE, International Conference on Pharmaceutical and Biomedical Engineering, June 12-13, 2017 Taipei, Taiwan, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE, Global Pharmaceutical Regulatory Affairs Summit,24 - 26 October 2017,Prague, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
Regulatory Affairs Professionals Society (RAPS), Association of Clinical Research Professionals - ACRP, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Association of Clinical Research Organizations, Pharmaceutical Group of the European Union (PGEU), International Pharmaceutical Federation (FIP), European Association of Employed Community Pharmacists in Europe (EPhEU),
Track 10: Pharmacovigilance
It deals with prevention of adverse drug reaction by collection, detection, assessment, monitoring. It concerns with the identifying hazards associated with pharmaceutical formulation. The advancement associated with pharmacovigilance helps in producing the valid formulation by focussing on the collected data from patients and health care providers. U.S. pharmacovigilance market size was valued over USD 1 billion in 2015, and predicted to witness 10.7% CAGR from 2016 to 2024 to surpass USD 2.5 billion by 2024.
Related Pharmaceutical Events| Pharmaceutical Conferences
12th Annual Pharma Middle East Congress, Sep 25-27, 2017 Dubai, UAE, 4th Annual Congress on Drug Discovery & Designing, July 03-05, 2017 Bangkok, Thailand, 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia, 7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE
Related Societies
International Society of Pharmacovigilance (ISOP), Pharmaceutical Information and Pharmacovigilance Association (Pipa), AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Drug Discovery and Development Interface (DDDI), European Pharmaceutical Union, Pharmaceutical Society of Australia, Canadian Pharmacists Association
Track 11: Good Manufacturing Practices
GMP is Set of guidelines to manufacturers must meet to assure that the products are high quality and safe. GMP covers all aspects of production from the starting to end of the manufacturing aspects. Introduced latest trends in GMP are risk- based approaches which are to encourage adoption of new technology by pharmaceutical industry.
Pharmaceutical industries are facing hurdles in adopting new technologies quickly.
Related Pharmaceutical Events| Pharmaceutical Conferences
9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, International Conference on Biotech Pharmaceuticals, October 23-25, 2017 Paris, France, 12th Annual Pharma Middle East Congress, Sep 25-27, 2017 Dubai, UAE, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, Pharmaceutical regulatory summit, March 28-31,2017 China, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
European Medicines Agency, Canadian Pharmacists Association, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Indian Pharmacist Association, Pharmaceutical Group of the European Union (PGEU), International Biopharmaceutical Association, European Biopharmaceutical Enterprises
Track 12: Ethics In Pharmacy
Pharmacy is a healthcare profession assisting individual in making the best use of medicine. Ethics are intended to state publicly the principles that form the fundamental basis of the roles and responsibilities of pharmacists. Spending on specialty drugs in 2012 in the United States was about $87 billion. Estimates suggest that it could quadruple by 2020, reaching about $400 billion
Related Pharmaceutical Events| Pharmaceutical Conferences
6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 Chicago, Illinois, USA, 10th Asia-Pacific Pharma Congress May 08-10, 2017 Singapore, 5th International Conference on Clinical Pharmacy, October 23-24, 2017 Orlando, Florida, USA, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore,7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
American Society for Pharmacy Law, European Pharmaceutical Union, Pharmaceutical Society of Australia, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), American Society of Consultant Pharmacists (ASCP), American Society of Consultant Pharmacists Foundation
Track 13: Pharmacy & Pharmacist
Pharmacists are becoming a more integral part of the health care team. Pharmacists do indeed dispense medicines, but first they check for any possible interactions with other medicines or medical conditions. They also instruct patients on how to take the medicines and will inform them about what to do if certain side effects arise. Pharmacy is the healthcare service science and expertise of preparing and dispensing drugs. Pharmacy Modern service includes more traditional and advanced compounding and dispensing medication healthcare.The biggest challenge facing pharmacy is continuing to communicate its role, deliver its value, and be reasonably reimbursed.
the Bureau of Labor Statistics projects a slower-than-average 3 percent employment growth for pharmacists by 2024, with the field adding 9,100 new jobs. Employment of pharmacists is expected to decline slightly in traditional retail settings because more people are having their prescriptions filled online or through mail order.
Related Pharmaceutical Events| Pharmaceutical Conferences
5th International Conference on Clinical Pharmacy, October 23-24, 2017 Orlando, Florida, USA, 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia, 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 Chicago, Illinois, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom, 19th International Conference on Pharmacy and Pharmaceutical Sciences, January 30 - 31, 2017, Dubai, UAE, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE
Related Societies
Canadian Pharmacists Association, American Society of Consultant Pharmacists (ASCP), American Society of Consultant Pharmacists Foundation, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU), Indian Pharmacist Association, European Pharmaceutical Union, Pharmaceutical Society of Australia
Track 14: Drug Disposition & Biological Products
Pharmacokinetic studies are more important for therapeutic biologics. Identifying the key factors that govern absorption, distribution, metabolism, and excretion of biologics as therapeutic agents improved the opportunities for the scientists working in drug disposition research field. The global ortho biological products market is anticipated to grow to $15.4 billion during 2016- 2022
Related Pharmaceutical Events| Pharmaceutical Conferences
3rd International Conference on Biopharmaceutics and Biologic Drugs, June 19-20, 2017 Philadelphia, USA, 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia, European Biopharma Congress, November 16-17, 2017 Vienna, Austria, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
International Alliance for Biological Standardization (IABS), International Biopharmaceutical Association, European Biopharmaceutical Enterprises, Association of Clinical Research Professionals - ACRP, Association of Clinical Research Organizations, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 15: Pharma Marketing
A pharmaceutical company is a commercial business licensed to research, develop, market and/or distribute drugs, most commonly in the context of healthcare. The winds of change are blowing through and, in fact, revolutionizing logistics in the global pharmaceutical industry. The prime contributing factors are raising costs, growing risks, changing consumer needs and tightening regulations, globally. Pharmaceuticals represented a US$300 bn-a-year market globally as of 2015, the World Health Organization states. The global pharmaceutical market is expected to surpass US$400 bn by 2018, with the ten largest pharmaceutical companies collectively commanding about a third of the market.
Related Pharmaceutical Events| Pharmaceutical Conferences
3rd International Conference and Expo on Drug Discovery & Designing, September 25-27, 2017, Vienna, Austria, International Conference and Exhibition on Nanomedicine and Drug Delivery, May 29-31, 2017 Osaka, Japan, 3rd International Conference on Biopharmaceutics and Biologic Drugs, June 19-20, 2017 Philadelphia, USA, 7th International Conference on Drug Discovery and Therapy, February 15th - 18th, 2016, University of Sharjah, Sharjah, UAE, Pharmaceutical regulatory summit, March 28-31,2017 China, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
Pharmaceutical Management Science Association (PMSA), Medical Marketing and Media, International Pharmaceutical Federation (FIP), European Association of Employed Community Pharmacists in Europe (EPhEU), International Biopharmaceutical Association, AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 16: Bio-availability and Bio equivalence
Bio availability is the rate and extent of active drug absorbed and available for action. Assessment of “interchangeability” between the generic and the innovator product is carried out by a study of bioequivalence (BE). During formulation of new entities bio availability and bio equivalents are also prime parameters. Through the conduct of bioavailability and bioequivalence studies, Generic drug products results on average bioequivalence in drug absorption. Global market of generic drug products revenue in 2012 reached 269 usd billions, and is estimated to reach 518 USD billion by 2018. Generic drugs save consumers an estimated $8 to $10 billion a year at retail pharmacies. Even more billions are saved when hospitals use generics.
Related Pharmaceutical Events| Pharmaceutical Conferences
3rd International Conference on Biopharmaceutics and Biologic Drugs, June 19-20, 2017 Philadelphia, USA, 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia, European Biopharma Congress, November 16-17, 2017 Vienna, Austria, 5th Annual International Conference on Pharmacology and Pharmaceutical Sciences, September 25-26, 2017, Singapore, 7th Annual Pharmacovigilance & Risk Management Strategies, Jan 25- 27, 2017, USA, 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia, 6th Global Congress on Mass Spectrometry October 18-19, 2017 Osaka, Japan, 16th Annual Medicinal & Pharmaceutical Sciences Congress July 03-05, 2017 Kuala Lumpur, Malaysia, World Affordable Medicines Congress, 7th - 8th February 2017, Barcelona, Spain, 2nd Pharma Digital and Multichannel Marketing Boot Camp, 7th - 8th February 2017 Hilton San Francisco, CA, 12th Annual Biomarkers Congress 21st - 22nd February 2017, Manchester, United Kingdom
Related Societies
International Biopharmaceutical Association, European Biopharmaceutical Enterprises, Pharmaceutical Society of Australia, Canadian Pharmacists Association, Pharmaceutical Group of the European Union (PGEU), AACP – American Association of Colleges in Pharmacy, Royal Pharmaceutical Society, Pharmaceutical society of Australia, National Pharmacy Association, Canadian Pharmacists Association, European Pharmaceutical Union (EPU)
Track 17: Entrepreneurs’ investment meet
The life sciences sector’s growth correlates highly with countries’ general economic strength and health care spending levels, and both of these vary widely around the globe. The European Union (EU) first approved a biologic in 2006, now there are more than 700 biosimilars approved or in the pipeline globally. Development and sales of biosimilars, biologic products which are similar but not identical to reference/originator biologic products, are beginning to accelerate. Analysts expect the worldwide biosimilars market to reach $25 billion to $35 billion by 2020. Generics have larger share of total global medicine spend, increasing from 27 percent ($261 billion) in 2012 to 36 percent ($421 billion) by 2017. Estimated global medical technology (medtech) segment revenues are expected to increase from $369 billion in 2015 to $454 billion in 2019, growing an average of 4.1 percent annually.
Over the last few decades a great deal of progress has increased in research oriented approaches like improved technologies, developed infrastructures, and increased research in the field of Drug discovery, clinical trials, Drug delivery, preformulation studies and Nano-medicine in the pharmaceutical industry. To develop a new pharmaceutical formulation and to conduct clinical trials it cost at € 1,926 million in 2016. The global pharmaceutical excipients market is projected to reach USD 8.1 Billion in 2021. The new drug delivery system demand is increasing due to greater understanding of the functional benefits of excipients, pharmaceutical industry growth, and patent expiries of several popular drugs are positively impacting the overall growth of the market.
Revenue of the worldwide pharma market in 2016 was $1072bn. The global pharmaceutical industry was worth $10613bn in 2015. The generic pharma industry is currently worth an estimated $ 225 billion US and the largest four generic pharma companies worldwide by sales (Teva, Sandoz, Mylan, & Watson) account for nearly 50% of generic prescriptions in the US and 40% worldwide. The United Sates is a major hub for drug manufacturing and its market imports were valued over $86 billion in 2016, making the world’s largest importer of pharmaceuticals and $47 billion exports.
According to the 2015 EU Industrial R&D Investment Scoreboard the pharmaceutical and biotechnology sector amounts to 18.2% of total business R&D expenditure worldwide.On the other side Europe drug manufacturing is 225000 €millions and exports were 361500 €millions. Europe spent 31,500 €millions on R&D in 2015 while US spent 47,061€millions.However, considering the all aspects the European pharma market is expected to grow by 27% between 2015 and 2022.
Germany is forecast to have highest increase in market value at € 11.4bn. Novartis is forecast to reduce its R&D spending, from $10.5bn in 2020 to only $9.2bn in 2022. Of the top 20, Celgene and Regeneron are forecast to grow their R&D expenses most rapidly, with R&D spend forecast to increase 11% per year until 2022. Overall, total R&D spends is expected to increase by 2.8% each year, reaching $182bn in 2022.
Patient awareness regarding adverse drug events is stimulating global pharmacovigilance market growth by increase in number of National pharmacovigilance centers. Spain pharmacovigilance market size was valued over USD 230 million in 2015 and witness 10.2% CAGR from 2016 to 2024, to surpass USD 550 million by 2024.
Drug discovery technologies study includes Bio analytical Assays, Bioinformatics, Cell Based Assays, Genomic Technologies, High Throughput Screening, Proteomic Technologies and Others comprising Epigenetics, Metabolomics, Combinatorial Chemistry, Synthetic Biology, Systems Biology and Nanotechnology. The global market value for these technologies market with an expected share of about 30% in 2015 valued at $18 billion followed by Bioanalytical Assays or instruments by about $14 billion.
Innovative formulations are estimated to have contributed to 73%. Biopharmaceuticals have set new standards for popular drugs, which are traditionally defined as drugs that have $1 billion or more in annual sales; the top 15 biopharmaceutical products each profits annual revenue of more than $2 billion, with some drugs generating sales of more than $10 billion a year. In 2015 the top NMEs include Pfizer’s Ibrance, J&J’s Darzalex and Gilead’s Genvoya with a cumulative value of $32bn of sales expected in 2020. The increasing number of breakthrough therapy approvals also proves the ability of the pharmaceutical R&D to offer incremental benefits to targeted patient segments with larger unmet needs.
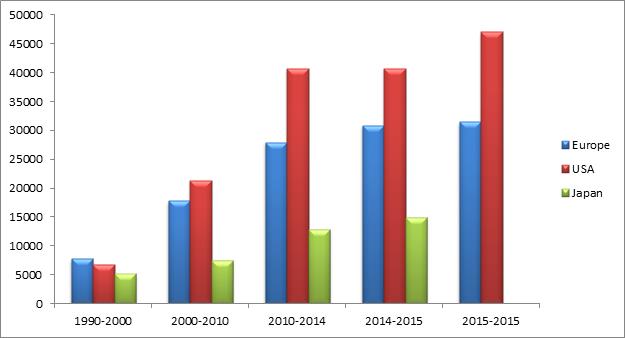
Pharmaceutical R&D Expenditure In Europe, USA and Japan (Millions Of National Currency Units*), 1990–2016
The market across US, Europe and Japan has accelerated by 7.5% in 2016.The graph illustrates the respective countries investments in R&D, Europe investing in a consisting manner along with other countries and competing with investment of USA. Japan’s investment is gradually increasing but less than other countries. The North American market (USA & Canada) remained the world’s largest market with a 48.7% share, well ahead of Europe and Japan.
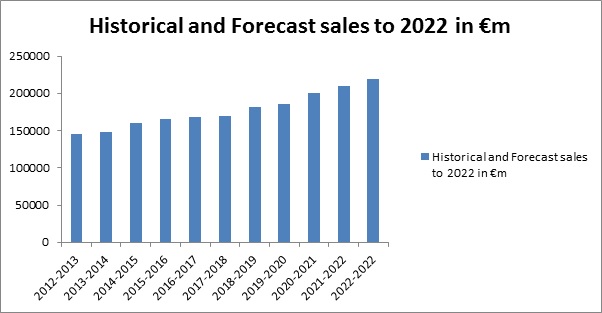
European pharma market perspective by 2022
Europe is now facing increasing competition from emerging economies, rapid growth in the market and research environments from other countries. On the contrary, the sector facing real challenges in regulatory hurdles and escalating R&D costs, the sector has been severely hit by the impact of fiscal measures introduced by governments across much of Europe since 2010. The research–based pharmaceutical industry can play a critical role in restoring Europe to growth and ensuring future competitiveness in an advancing global economy. EU pharma market fragmentation resulted in a trade was estimated to amount of € 5,589 million (value at ex–factory prices) that benefit neither social security nor patients and deprived the drug development industry to fund new innovation in formulation.
Parallel trade was estimated to amount to € 5,589 million. In 2015 North America accounted for 48.7% of world pharmaceutical sales compared with 22.2% for Europe. According to IMS Health data, 58% of sales of new drug discovery made during the period 2010–2015 were on the US market, compared with 23% on the European market. Europe is ensuring competitive growth along with other countries. By 2022, products currently in drug development will account for 17% of sales in Europe. Europe is expected to show a 3% CAGR 2016-22, mainly driven by the launch of new products currently in development. The penetration of biotech products is set to increase from a 24% market share in 2015 to 29% in 2022.
Pre-Clinical trial will witness a sustainable 10.3% growth, with target market size slated to exceed USD 84 million by 2024. These studies are very critical and are mandatorily required before human clinical trials to collect safety and toxicity data in support of the new treatment. Phase III clinical trial should grow from over USD 492 million in 2015 to over USD 1,254 million by 2024, witnessing 10.9% CAGR from 2016 to 2024, owing to growing need for drug safety monitoring and evaluate drug efficacy based on risk-benefit ratio. Phase IV clinical trial market size was over USD 2.4 billion in 2015, growing at 10.9% from 2016 to 2024, owing to increasing drug safety concerns and growing public health awareness about adverse drug events. It aims at continuous safety surveillance through adverse drug monitoring as long as a product is marketed.
Despite of all parameters Europe has to meet parameters of Regulatory affairs and acts that lies at the basis of European pharmaceutical ethics and all of its intellectual property rights and its complications.
Conference Highlights
- Pharmaceutical Sciences
- Drug Discovery & Research
- Drug Therapy
- Advances in Clinical Trials
- Drug Delivery Systems
- Pharmaceutical Drug Formulations
- Innovations in Drug Development
- Regulatory Affairs & Intellectual Property Rights
- Pharmacovigilance and Drug Safety
- Good Manufacturing Practices
- Ethics In Pharmacy
- Pharmacy & Pharmacist
- Drug Disposition & Biological Products
- Pharmaceutical Analysis
- Pharma Marketing
- Bio-avilability and Bio-equivalence
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 16-18, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutical Care & Health Systems
- Clinical Pharmacology & Biopharmaceutics
- Journal of Developing Drugs
Abstracts will be provided with Digital Object Identifier by


















































































































